
If you use nothing else on your skin, I know you do use a cleanser! There is a huge array of products out on the market, with many different price points, different additives and different formulations. Here I’ll be reviewing how soap and cleansers work to clean your skin. Pretty much all how soap and cleansers work this way, whether it is Dawn Dish Soap or a super expensive facial cleanser from Chanel. Quick, read the epidermis post if you haven’t yet (or re-read it if you have, I added a few things) before we start!
How Soap Works
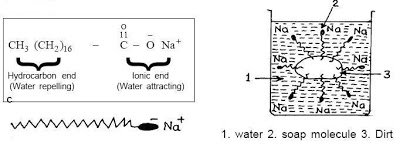
All soap and cleansers work the same way, whether a $1 bar of soap or a $100 bottle of La Mer cleanser. Really! In order to both get rid of oils, sweat and dirt on the skin while making the whole thing water soluble, surfactants are used. Basically, look at the left side of the picture above. This is the basic soap molecule. There is a long chain of non-polar (neutral) atoms (that part that looks like a tail) and this part is attracted to the dirt, oil, etc. The other end (the polar, or charged end) is attracted to water. So, lots of little soap molecules will surround the piece of dirt, oil or whatever; see the right side of the picture. Note how they surround the offensive bit with their non-polar end, and the polar (water attracting) end is out into the water, allowing the whole thing to be water soluble.
Note that not all cleansers are technically “soap.” There are soaps and detergents, the difference is the way in which they are made, in addition to their pH. Soaps tend to be more alkaline, and as such may result in more drying and irritation of the skin. Both work through the surfactant mechanism I just described, and there are different fats and additives that compose the rest of the cleaners.
The whole thing sounds pretty simple, doesn’t it? Well, there are definite downsides to this whole process.
1. Redness and Irritation: The soap molecules may surround and damage the Keratin within the skin. This allows the Keratin to swell and become overhydrated, damaging the skin and it’s barrier function. In turn, this allows the soap to penetrate more deeply into the skin, where they interact with nerve endings and are attacked by the body’s immune system, resulting in irritation, redness and itching.
2. Dryness: The soap molecules may remove some of the lipid between the cells of the Cornified Layer, and may also remove some of the moisturizing molecules within the cells of this layer as well.
3. Tightness after Washing
The extent to which a soap is irritating depends upon several thing- the potency of the surfactant, the pH of the soap (higher pH=more basic/alkaline=more irritating, irritating soaps will be in the range of 9.5-11), and rinsability. All of these effects are minimized by adding moisture through depositing lipids and humectants (things that attract moisture) into the skin. As well, artificially created surfactants (also called syndets) are less harsh on the skin than more naturally created surfactants.
Types of Cleansers
There are many fomulations of cleansers out of the market today, and often the only way to discover what type of cleanser you have is to check the label!
Most soaps can be easily divided in terms of their composition, I find this the easiest way to think of them.
1. Soap: Old fashioned soap, pH is usually 9-10
2. Combars: Combines soap with “surface active agents” with a pH 9-10
3. Syndet: Synthetic detergents rather than than mostly soap surfactants, they usually are<10% soap, and have a pH of 5-5.5 which means they are often more gentle on your skin.
Soaps are also commonly divided by how they are made, especially those that are in bars. I’m not going into the whole process or explanation, but you can read a pretty decent explanation of it here on Wikipedia.
Considerations for Certain Forms of Cleansers
1. Liquid: When liquid soaps first came out, they were quite the revolution in the cleansing industry. Their formulas allow milder surfactants and more (and better quality) moisturizers to be used.
–Emollient-Rich vs. Humectant-Rich: Both are forms of moisturizers, however humectants simply attract moisture to the epidermis, and are easily washed off of the skin. Emollients help to fill in the spaces between the corneocytes in the cornified layer, and as they are not water soluble, they are not easily washed away. Emollient rich body washes are made by Dove and Olay.
2. Bar: Bar soaps are typically more aggressive in terms of their surfactants. This means that not only will they do a better job of removing soap and oils, they also will be more likely to irritate and dry out the skin.
3. Lipid free cleansers: A liquid cleanser that cleans without fats, it removes dirt and makeup easily and leaves behind a thin film. Often used by those with sensitive skin.
Usually contains: water, glycerin, cetyl alcohol, stearyl alcohol, sodium laurel sulfate (a detergent), and propylene glycol (can cause stinting).
4. Cleansing Creams/Cold Creams: They seem old fashioned I know, but they are pretty great for dry skin. Usually contain water, mineral oil, petrolatum, and waxes, they use a borax derived detergent (decahydrate of sodium tetraborate)
5. Body Washes: Depends upon a little plastic poof to add water and air to disperse the product (you know the one I mean, it’s hanging in your shower right now), they are made of synthetic detergents (therefore less harsh on the skin) and they can have high amounts of petrolatum for hydration (Oil of Olay’s body wash has 17%, I heard from a dermatologist that this is the highest % on the market).
6. Low-foaming Washes: Often used to wash the face, often they have little to no surfactant, but do not contain moisturizers such as humectants or emollients. Cetaphil fits into this category.
Common Cleanser Ingredients
Pretty much all surfactants are made from an oil/fat which reacts with a base, producing the surfactant molecules you saw in the pic above. The length of the carbon chain “tail” depends upon which oil you started with, for example those with 10-14 carbons in the chain are more aggressive and more irritating (these are mostly derived from coco oil).
How to read an Ingredients list at The Beauty Brains
| Purpose | Ingredient | |
|---|---|---|
| Surfactants | Natural soap | synthetic surfactants |
| Preservative/Antioxidants | (BHT) butyl hydroxy toluene | 2-t-butylbenzene-1 |
| Detergents | sodium cocoate sodium tallowate sodium palm kernelate sodium stearate sodium palmitate triethanolamine stearate sodium cocoyl isethionate sodium isethionate |
sodium dodecyl benzene sulfonate sodium cocoglyceryl ether sulfonate sodium laureth sulfate- a harsher surfactant cocoamido propyl betaine lauric acid diethenolamine (lauramide DEA), sodium cocoyl isethionate disodium laureth sulfosuccinate Decahydrate of sodium tetraborate Ammonium Lauryl (or Laureth) Sulfate – a milder surfactant |
| Preservative/Sequestering agent (keep hard minerals in the water) | (EDTA) ethylene diamine tetraacetic acid (EHDP) hydroxyethane diphosphonic acid, also known as editronic acid |
Complex phosphates and sodium citrate |
| Whitening agent | Titanium dioxide | |
| Processing aids | Salt | |
| Fillers | Starch, clays, salts, etc. | |
| Lather Boosters | Co-surfactants (e.g., SLES and betaine) | sodium carboxymethylcellulose |
| Antibacterial Agents | Benzoyl Peroxide Sulfur |
Resorcinol antibacterials –triclocarban- attacks gram + bacteria –triclosan- attacks gram + and – bacteria |
| Moisturizers/Skin Feel Agents | Polyols Glycerin |
lanolin petrolatum |
| Humectant-Attract water from the dermis into the epidermis, and from the air in humidity >70% | Acetamide MEA Agarose Ammonium lactate Arginine PCA Betaine Butylenes glycol Cocamidopropyl Betaine Copper PCA Corn glycerides Diglycereth-7 malate Diglycerin Dimethyl imidazolidinone Erythritol Gelatin Glucose Glucuronic acid Glucuronolactone Glutamic acid Glycereth-12 Glycerin Honey extract Hylauronic acid Hydrololyzed wheat starch Hydroxyethyl sorbitol Lactamide Lactic acid Maltitol |
Melibiose Mineral oil Panthenol Pantolactone PCA Polyglucuronic acid Propylene glycerol Saccharide hydrolysate Sea salt Seasame amino acids Sodium aspartate Sodium lactate Sodium malate Sodium PCA Sodium polyaspartate Sorbitol TEA-lactate Triglycereth-7 citrate Urea Xylose |
| Emollient | Emollients help to fill in the spaces between the corneocytes in the cornified layer, they remain in the layer Acetylated lanolin Acetyl trihexyl citrate Avocado sterols Butyl myristate C 14-15 alcohols C 12-13 alkyl ethylhexonoate Caprylyl glycol Castor oil Cetyl acetate C 14-16 glycol palmitate C 12-20 isoparaffin Cyclomethicone Decyl oleate Diethylhexyl adipate Diethylhexyl malate Diisodecyl adipate Diisopropyl dilinoleate Dimethicone copolyol Dipropyl adipate Ethylhexyl palmitate Ethyl linoleate Glyceryl dioleate Glyceryl ricinoleate Glyceryl stearates Glycol palmitate (palm oil) Glycine Soja (soybean oil) Glycol stearate |
Helianthus Annuus (sunflower) Seed oil Hexyl laurate Isocetyl alcohol Isodecyl steararate Isohexyl palmitate Isopropyl isostearate Isopropyl myristate Isopropyl palmitate Isosteryl alcohol Jojoba oil Lanolin Methyl palmitate Myristyl propionate Octyl octanoate Octyl stearate PEG-4 lanolate PEG-5 tristearyl citrate Polyglyceryl-6 oleate Plyclycerol-2 triisosterate PPG-20 cetyl ether PPG-4 laureth-2 Propylene glycol linoleate Squalene Sucrose oleate Sunflower seed oil glycerides Tall oil glycerides Tridycyl stearate Wheat germ glycerides |
| Occlusives-Greasy ingredients that help to slow the evaporation of water from the skin, they are most effective when applied to damp skin | Acetylated Castor Oil Acetylated lanolin alcohol Behenyl isosterate Beeswax Canola oil Caprylic/capric triglyceride Carnauba Cetearyl methicone Cetyl ricinoleate Cholesteryl oleate CyclomethiconeDecyl myristate Dimethicone Disteryl ether Glycol dioleate Hexyldecyl isostearate Hydrogenated castor oil Hydrogenated lanolin Isocetyl myristate Lanolin linoleate Lauryl Cocoate Lecithin |
Mineral oil- very frequently used due to it’s pleasant texture Myristyl myrisstate Neatsfool oil Octyldodecyl stearate Oleyl linoleate Palm kernal wax Paraffin Petaerythrityl tetracocoate Petroleum Propylene glycol dioleate Shark liver oil Soybean lipid Stearyl stearate Squalane Tall oil Tocopherol Trihexyldecyl citrate Triisosterin Vegetable Oil |


 I’m a doctor, a mommy and a bit of a beauty addict. If you let me, I can take 2 hours to get ready in the morning. Really. I'm on a quest for faster beauty that works!
I’m a doctor, a mommy and a bit of a beauty addict. If you let me, I can take 2 hours to get ready in the morning. Really. I'm on a quest for faster beauty that works!
Thanx for a very informative post! I am going to bookmark it for future reference.
Really such a nice and very impressive post. I was searching for this and got a total information in your post.
Thanks a lot.
Stacey Sharp
Pro argi 9